Isolated Cardiac Sarcoidosis Manifesting with Ventricular Tachycardia, Systolic Heart Failure, and Pre-Excitation Accessory Pathway
Isolated Cardiac Sarcoidosis Manifesting with Ventricular Tachycardia, Systolic Heart Failure, and Pre-Excitation Accessory Pathway
Author: Charles Ebersbacher, DO, MBA, Additional Authors**
See the poster presentation | See the poster
Introduction/Objective
Isolated cardiac sarcoidosis (iCS) accounts for substantial morbidity and mortality and is likely underdiagnosed. Some data suggest patients with iCS have worse outcomes, perhaps due to delayed diagnosis. We present a case of iCS manifesting with ventricular tachycardia, cardiomyopathy, and with a pre-excitation accessory pathway.
Case Presentation
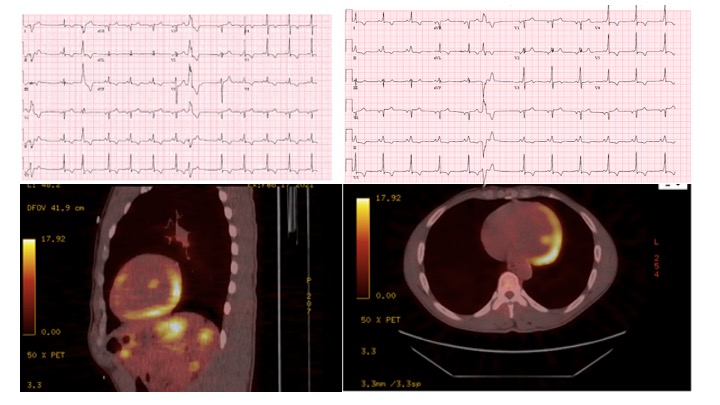
A 43-year-old African American male with history of tobacco use presented with multiple episodes of presyncope. Electrocardiogram (ECG) revealed junctional narrow QRS rhythm, inferolateral T-wave inversions, and premature ventricular contractions (PVCs). Cardiac monitoring showed multifocal PVCs and non-sustained ventricular tachycardia (NSVT). Echocardiogram showed biventricular systolic dysfunction. Coronary angiogram showed no evidence of obstructive coronary artery disease. Cardiac Magnetic Resonance (cMR) imaging showed significant delayed enhancement and edema in the inferior and inferolateral sub-epicardial and mid- myocardial wall segments. Positron Emission Tomography (PET) scan was markedly positive for fluorodeoxyglucose (FDG) uptake in the basal and mid inferior/inferolateral walls extending toward the apex, consistent with active cardiac sarcoidosis (CS). No extracardiac FDG uptake was seen on PET. The patient was started on high dose corticosteroids, and guideline directed medical therapy for systolic heart failure was initiated and slowly up-titrated. Methotrexate was later added based on persistent active inflammation on follow-up PET 2 months later. Ambulatory cardiac monitor showed, in addition to frequent NSVT and PVCs, multiple runs of narrow complex tachycardia with evidence of pre-excited sinus rhythm. Accessory pathway was later confirmed on electrophysiological study and successfully ablated, and the patient received an implantable cardioverter defibrillator (ICD). Six months later, the patient has no limiting heart failure symptoms, presyncope, and has reduced frequency of ventricular arrhythmias. He is slowly being tapered from corticosteroids, and he is scheduled for repeat PET to assess response to therapy. Ablation of NSVT is also planned if increased arrythmia burden persists after controlling active disease.
Discussion
Diagnosing iCS is challenging. Clinical suspicion and ruling out other diagnoses are key in reaching the diagnosis. Confirmation often relies on non-invasive imaging studies, as endomyocardial biopsy, the gold-standard, lacks sufficient sensitivity. Important cMR findings include patchy delayed gadolinium enhancement in the epicardial and mid-endocardial regions in a non-vascular distribution indicating scarring or active inflammation. Active inflammation, indicative of active cardiac sarcoidosis, is then confirmed by showing myocardial edema on T2 imaging, though this lacks sensitivity. PET scanning, however, is more sensitive in detecting active disease and serial PET can also show response to therapy but can miss disease in remission. PET can also identify extracardiac involvement of sarcoidosis. Therefore, combining the two imaging modalities is encouraged when establishing the diagnosis of cardiac sarcoidosis.
Accessory pathways often become clinically evident in patients with cardiac sarcoidosis, possibly due to the involvement of the atrioventricular node and His-Purkinjie system. These pathways can trigger more tachyarrhythmia and often require targeted ablation therapy.
Conclusion
Isolated cardiac sarcoidosis is an elusive diagnosis that requires high level of suspicion and multimodal imaging techniques to confirm the diagnosis. Further studies are needed to develop and validate a diagnostic algorithm and management approach for these patients.
**Additional Authors:
Charles Ebersbacher, DO, MBA, Case Western Reserve University, MetroHealth, Cleveland, OH
Ashten Gibson, DO, Case Western Reserve University, MetroHealth, Cleveland, OH
Soufian Almahameed, MD, Case Western Reserve University, MetroHealth, Cleveland, OH
Khalil Murad, MD, Case Western Reserve University, MetroHealth, Cleveland, OH